OVERVIEW
First Wave BioPharma’s product portfolio is built around its three proprietary technologies – latiglutenase, a potentially first-in-class oral biotherapeutic designed to aid gluten digestion and potentially reduce symptoms of celiac disease, capeserod, a selective 5-HT4 receptor partial agonist being developed for gastroparesis, and adrulipase, a recombinant lipase designed to enable the digestion of fats and other nutrients in patients with exocrine pancreatic insufficiency.
Latiglutenase
First Wave is developing latiglutenase as an orally administered, recombinant digestive enzyme therapy to treat celiac disease as an adjunct to a gluten-free diet.
CAPESEROD
First Wave is developing capeserod, a selective 5-HT4 receptor partial agonist designed to promote gastric motility as a treatment for gastrointestinal indications, including gastroparesis.
ADRULIPASE
First Wave is advancing two programs built around adrulipase for the treatment of exocrine pancreatic insufficiency (FW-EPI) in patients with cystic fibrosis (CF) and chronic pancreatitis (CP).
Adrulipase is a recombinant lipase enzyme for the treatment of exocrine pancreatic insufficiency (EPI) associated with cystic fibrosis (CF) and chronic pancreatitis (CP). Adrulipase, supplied as an oral, non-systemic, biologic capsule, is derived from the Yarrowia lipolytica yeast lipase and breaks up fat molecules in the digestive tract of EPI patients so that they can be absorbed as nutrients.
EPI is a condition characterized by deficiency of the exocrine pancreatic enzymes, resulting in a patient’s inability to digest food properly, or maldigestion. The deficiency in this enzyme can be responsible for greasy diarrhea, fecal urge and weight loss.
The digestive standard of care for both CF and chronic pancreatitis (CP) patients with EPI are commercially-available PERTs. Ideally, a stable daily dose of PERT will enable CF patients to eat a normal to high-fat diet and minimize unpleasant gastrointestinal symptoms. In practice, however, a substantial number of CF patients do not achieve normal absorption of fat with PERTs. Moreover, PERTs require the administration of as many as 40 pills per day and have potential issues with Black Box safety warnings.
In developing adrulipase, First Wave is seeking to provide CF and CP patients with a safe and effective therapy to control EPI that is non-animal derived and offers the potential to dramatically reduce their daily pill burden.
Capeserod is a selective 5-HT4 receptor partial agonist, which First Wave licensed from Sanofi and is repurposing and developing for GI indications. Sanofi previously conducted seven Phase 1 and two Phase 2 trials investigating the potential of capeserod for neurological disorders. In these trials, involving over 600 patients, Capeserod appeared safe and well-tolerated.
Sanofi’s research on capeserod determined the drug’s mechanism of action may directly or indirectly initiate the peristaltic or secretory reflex by releasing neurotransmitters that can decrease colonic transit time and improve bowel movement in constipation. Additionally, capeserod may reduce the likelihood of inflammation and damage of epithelial cells where its partial agonistic effect is expected via stimulation of 5HT4b in intestinal epithelium. Moreover, combination therapy with ADBR3 agonists may provide additive effect and relief in symptoms in ulcerative colitis.
Based on this research and subsequent artificial intelligence (AI)-empowered analyses, First Wave believes capeserod has potential applications for several gastrointestinal disorders in multibillion-dollar markets where there are significant unmet clinical needs. These GI indications include gastroparesis, a disease whereby the movement of food from the stomach to the small intestine is delayed, resulting in severe constipation, and pediatric ulcerative colitis, an inflammatory bowel disease (IBD) that causes irritation, inflammation, and ulcers in the lining of the colon.
Latiglutenase is an orally administered mixture of two gluten-specific recombinant proteases that degrades gluten proteins into small physiologically irrelevant fragments that is designed to be administered as an adjunct to a gluten-free diet (GFD).
In Phase 2a and 2b clinical trials, latiglutenase has been shown to mitigate gluten-induced intestinal mucosal injury as well reduce the severity and frequency of symptoms in celiac disease patients. Evidence of symptom relief was particularly pronounced for patients who continue to have positive serology to gluten-induced antibodies (seropositive) despite following a GFD as seen in the results shown below (Syage 2017, 2019). These studies also demonstrated that Latiglutenase is well tolerated by CD patients with no discernable difference in adverse event profile for active vs. placebo treated patients.
Figure 1: Percent reductions in symptom severity relative to placebo for the combined 600 mg and 900 mg treatment groups.
Additionally, in the CeliacShield™ trial (NCT03585478) conducted at Mayo Clinic (Murray 2022), the gluten-challenge trial measured several outcomes including histology, symptoms, serology, and gluten in urine. Histologic protection was assessed by measuring changes in villous height to crypt depth ratio (ΔVh:Cd) and intraepithelial lymphocytes (ΔIEL) before and after a 6-week, 2-g per day gluten challenge period. The attenuation of ΔVh:Cd and ΔIEL for the active (1200-mg latiglutenase) group relative to placebo was 88% and 60% with p-values of 0.0570 and 0.0181 (ANCOVA), respectively.
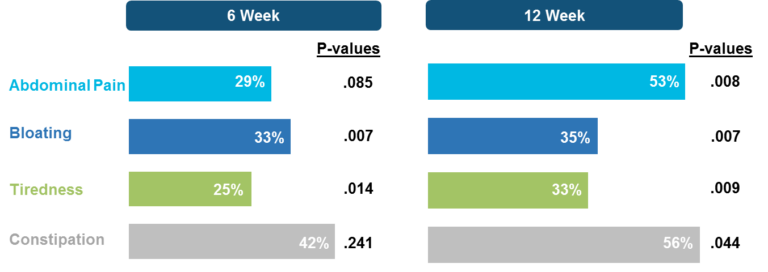
Measurements of gluten immunogenic peptides (GIP) in urine showed reduction of gluten of about 95% for latiglutenase vs. placebo with p < 0.0001 (Figure 2) demonstrating the mechanism of action. The reduction in symptom severity for latiglutenase relative to placebo ranged from 53% to 99% for abdominal pain, bloating, tiredness and the overall non-stool composite scale that also includes nausea. All of these symptoms showed consistent trends when monitored over three 2-week intervals with p values ranging from <0.001 to 0.030 (see Figure 3).
Figure 2. GIP concentration in urine before, during and after the gluten challenge treatment period
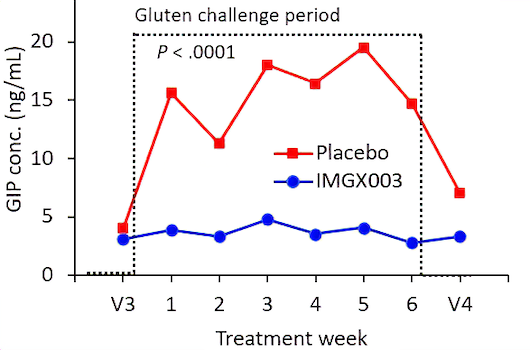
Figure 3. Mean change from baseline (% worsening) for non-stool composite for three 2-week gluten challenge treatment periods for latiglutenase vs. placebo.

PUBLICATIONS
|
|
LATIGLUTENASE: |
|
Murray JA, Syage, JA, Wu T-T, Dickason MA, et al. Latiglutenase Protects the Mucosa and Attenuates Symptom Severity in Patients with Celiac Disease Exposed to a Gluten Challenge, Gastroenterology 2022; 163:1510-1521. https://doi.org/10.1053/j.gastro.2022.07.071 Syage JA et al., Latiglutenase Treatment for Celiac Disease: Symptom and Quality of Life Improvement for Seropositive Patients on a Gluten-Free Diet. GastroHep 2019;1:293-301. https://doi.org/10.1002/ygh2.371 Syage JA, Murray JA, Green PHR, Khosla, C. Latiglutenase Improves Symptoms in Seropositive Celiac Disease Patients While on a Gluten-Free Diet. Dig Dis & Sci 2017;62:2428-2432. https://doi.org/10.1007/s10620-017-4687-7 Moron B, Verma AK, Khosla C et al. CYP3A4-catalyzed simvastatin metabolism as a non-invasive marker of small intestinal health in celiac disease. Am J Gastroenterol 2013;108:1-8. https://doi.org/10.1038/ajg.2013.151
Leffler D, Kupfer SS, Lebwohl B, Bugin K, Griebel D, et al., Development of Celiac Disease Therapeutics: Report of the Third Gastroenterology Regulatory Endpoints and Advancement of Therapeutics Workshop. Gastroenterology 2016;151:407–411. https://doi.org/10.1053/j.gastro.2016.07.025 Green PHR, Lebwohl B, Greywoode R. Celiac disease. J Allergy Clin Immunol 2015;135:1099-1106. https://doi.org/10.1016/j.jaci.2015.01.044 Kelly CP, Bai, JC, Liu E, Leffler DA. Celiac disease: clinical spectrum and management. Gastroenterol 2015;148:1175-1186. https://doi.org/10.1053/j.gastro.2015.01.044 Rubio-Tapia A, Murray JA. Celiac disease. Curr Opin Gastroenterol 2010;26:116–22. https://doi.org/10.1097/mog.0b013e3283365263 Schuppan D, Junker Y, Barisani D. Celiac disease: from pathogenesis to novel therapies. Gastroenterology 2009;137:1912–33. https://doi.org/10.1053/j.gastro.2009.09.008 |
|
|
ADRULIPASE: |
|
“Results from an Open-Label, Phase 2 STUDY with Escalating Doses of Adrulipase Alfa on top of a Stable Dose of Porcine Pancreatic Enzymes (PPE) in CF Patients Not Fully Compensated with PPE as Sole Therapy”. Konstan M, Karadag B, Srinivasan D, Pennington J. 2023 Digestive Disease Week (DDW) Presentation. "Formulation Development of Enterically Protected Spray Dried Dispersions of Adrulipase." Desai H, Conlon D, Brown A, Draganov A, Tagliaferri F, Srinivasan D, Brieger M, Stover, T. AAPS 2022 PHarmSCi 360 Conference Poster. “A Phase 2, Open-Label, Multicenter, 2×2 Crossover Trial to Assess the Safety and Efficacy of MS1819-SD in Patients with Exocrine Pancreatic Insufficiency Due to Cystic Fibrosis”. Konstan M, McBennett K, Boas S, Mazurek H, Gangal M, Srinivasan D, Pennington J. 2020 Digestive Disease Week (DDW) Presentation. “Impact of a spray dried recombinant lipase, MS1819, For the treatment of exocrine pancreatic insufficiency in patients with chronic pancreatitis. Results of a multicenter, Phase II, open-label, non-randomized study”. Nguyen, N, Lebreton, L, Smith G, Jais P, Schue M, Spoor, T. 2019 Digestive Disease Week (DDW) Presentation. “Yarrowia lipolytica Lipase 2 is Stable and Highly Active in Test Meals and Increases Fat Absorption in an Animal Model of Pancreatic Exocrine Insuffiency.” Aloulou A, Schué M, Puccinelli D, Milano S, Delchambre C, Leblond Y, Laugier R, Carrière F. Gastroenterology. December 2015. 149(7):1910-1919 |
|
|
NICLOSAMIDE: |
|
Marafini, I, De Crisofaro, E., Salavatori, S, Calabrese, E, Lolli, E, Monteleone, I, Franchi, L, Ciccopcioppo, R, Glick, G, Opipari, A, Monteleone, G. Niclosamide Enema for Active Distal Ulcerative Colitis: A Phase 1, Open-Label Study. Inflamm Bowel Dis. 2023 July 21. Read more. “Niclosamide Enemas, A Safe And Effective Treatment In Mild To Moderate Ulcerative Proctitis/Proctosigmoiditis: Results From An Open Label Study”. Marafini I, Monteleone G, Salvatori S, Ciccocioppo R , Opipari A, Pennington J , Piccirillo M.. 2022 Digestive Disease Week (DDW) Presentation. Shin, B., Benavides, G. A., Geng, J., Koralov, S. B., Hu, H., Darley-Usmar, V. M., & Harrington, L. E. (2020). Mitochondrial oxidative phosphorylation regulates the fate decision between pathogenic Th17 and regulatory T cells. Cell Reports, 30(6), 1898-1909. doi:10.1016/j.celrep.2020.01.022. Read more. Fuseini, H., Cephus, J., Wu, P., Davis, J. B., Contreras, D. C., Gandhi, V. D., Rathmell, J. C., & Newcomb, D. C. (2019). ERα signaling increased IL-17A production in Th17 cells by upregulating IL-23R expression, mitochondrial respiration, and proliferation. Frontiers in Immunology, 10. doi:10.3389/fimmu.2019.02740. Read more. Kaufmann, U., Kahlfuss, S., Yang, J., Ivanova, E., Koralov, S. B., & Feske, S. (2019). Calcium Signaling Controls Pathogenic Th17 Cell-Mediated Inflammation by Regulating Mitochondrial Function. Cell Metabolism, 29(5), 1104-1118. doi:10.1016/j.cmet.2019.01.019. Read more. Franchi, L., Monteleone, I., Hao, L., Spahr, M. A., Zhao, W., Liu, X., Demock, K., Kulkarni, A., Lesch, C. A., Sanchez, B., Carter, L., Marafini, I., Hu, X., Mashadova, O., Yuan, M., Asara, J. M., Singh, H., Lyssiotis, C. A., Monteleone, G., Opipari, A. W., & Glick, G. D. (2017). Inhibiting oxidative phosphorylation in vivo restrains Th17 effector responses and ameliorates murine colitis. The Journal of Immunology, 204(2). doi:10.4049/jimmunol.1600810. Read more. Tao, H., Zhang, Y., Zeng, X., Shulman, G. I., & Jin, S. (2014). Niclosamide ethanolamine improves blood glycemic control and reduces hepatic steatosis in mice. Nature Medicine, 20(11), 1263-1269. doi:10.1038/nm.3699. Read more |